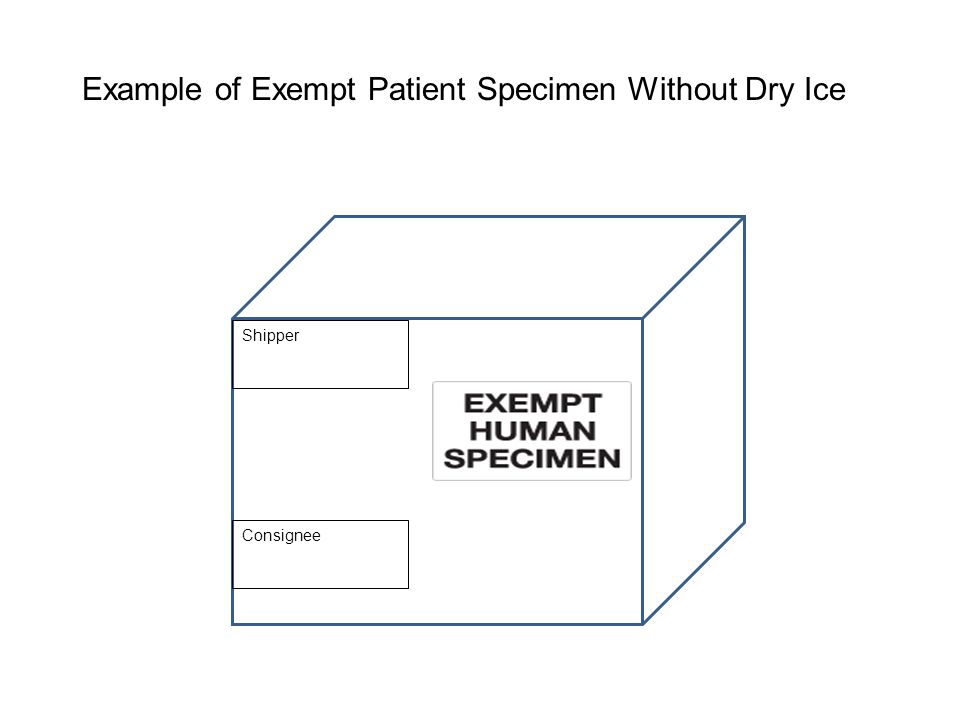
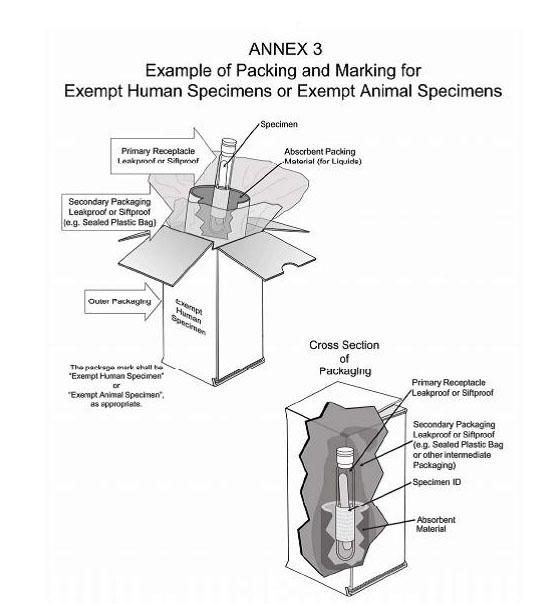
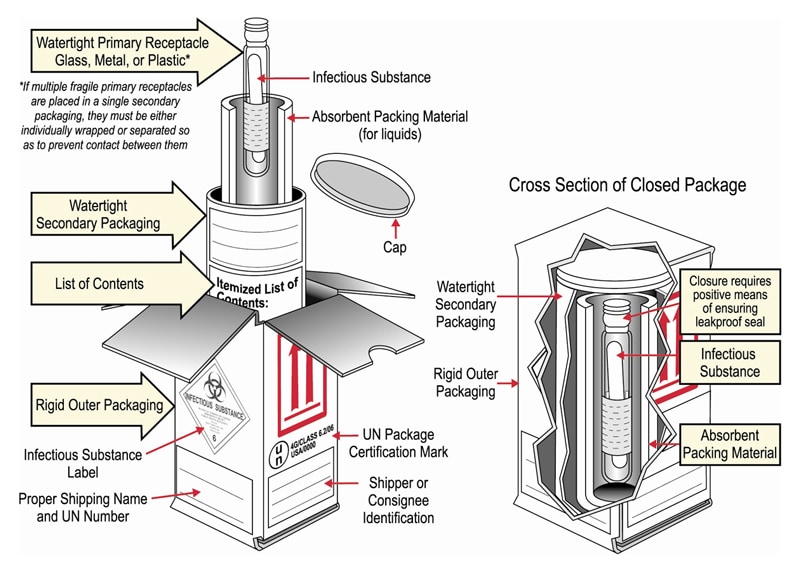
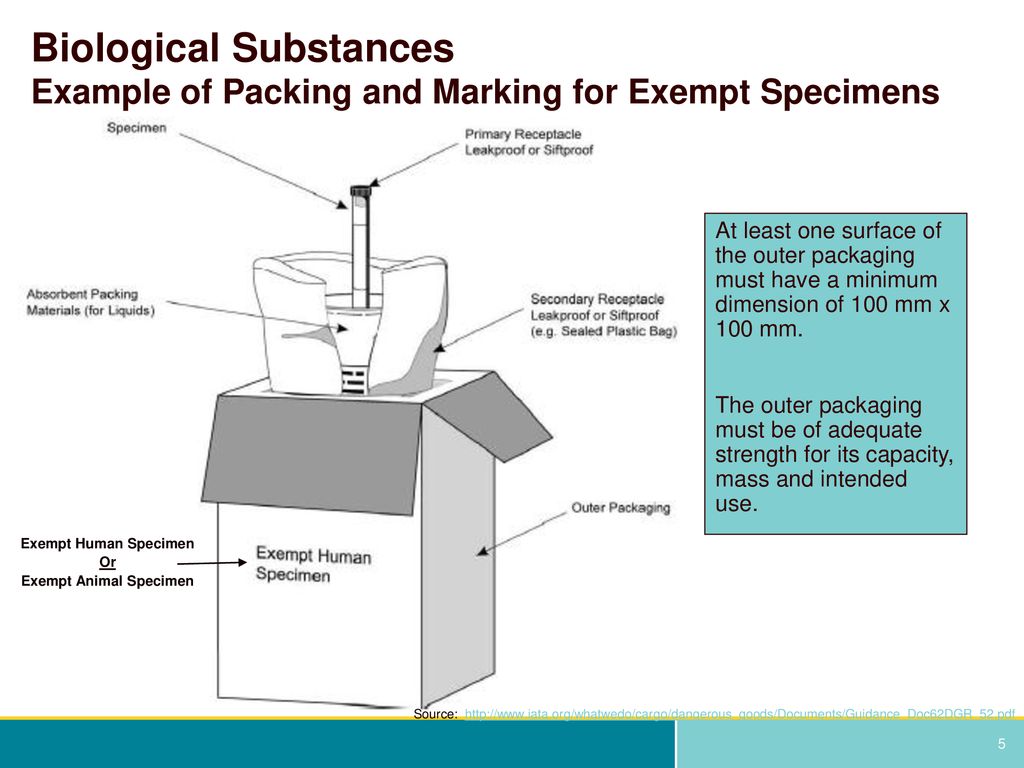
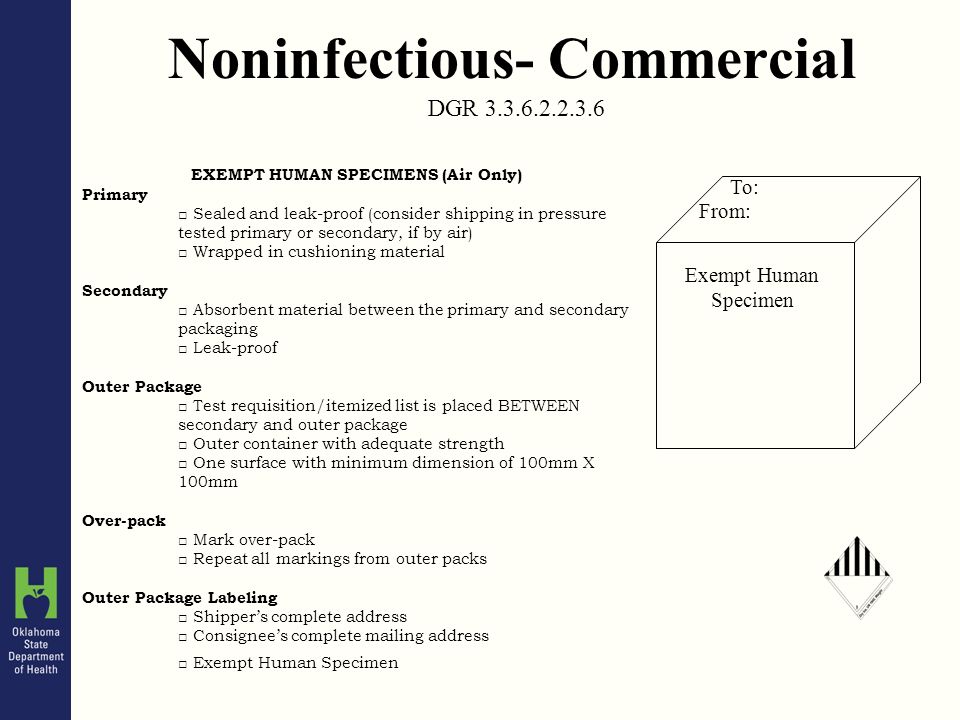
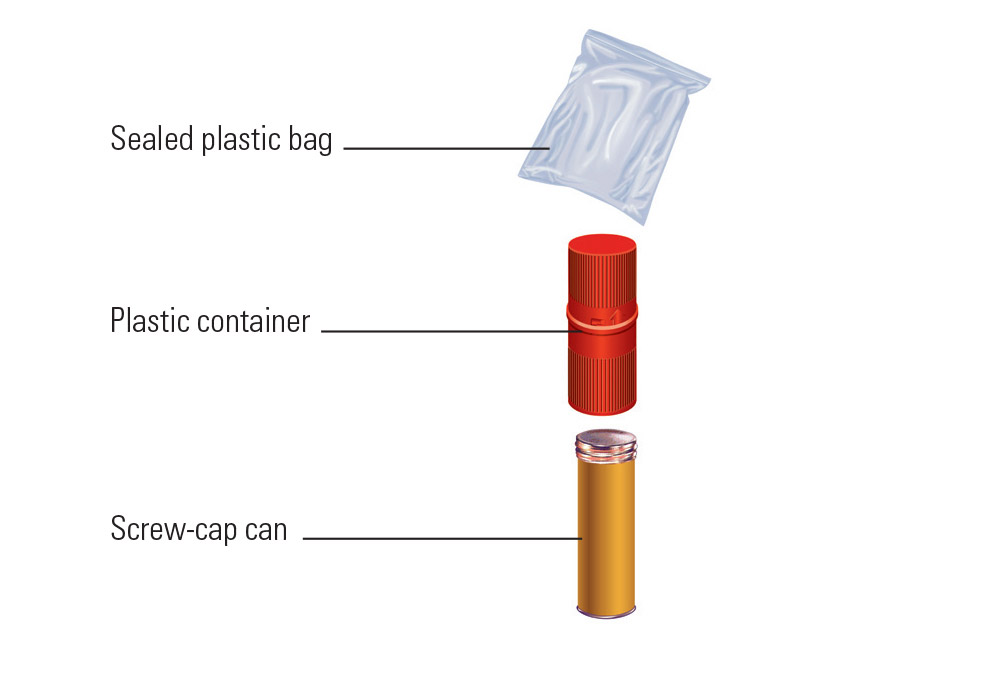
45 examples of exempt human specimens
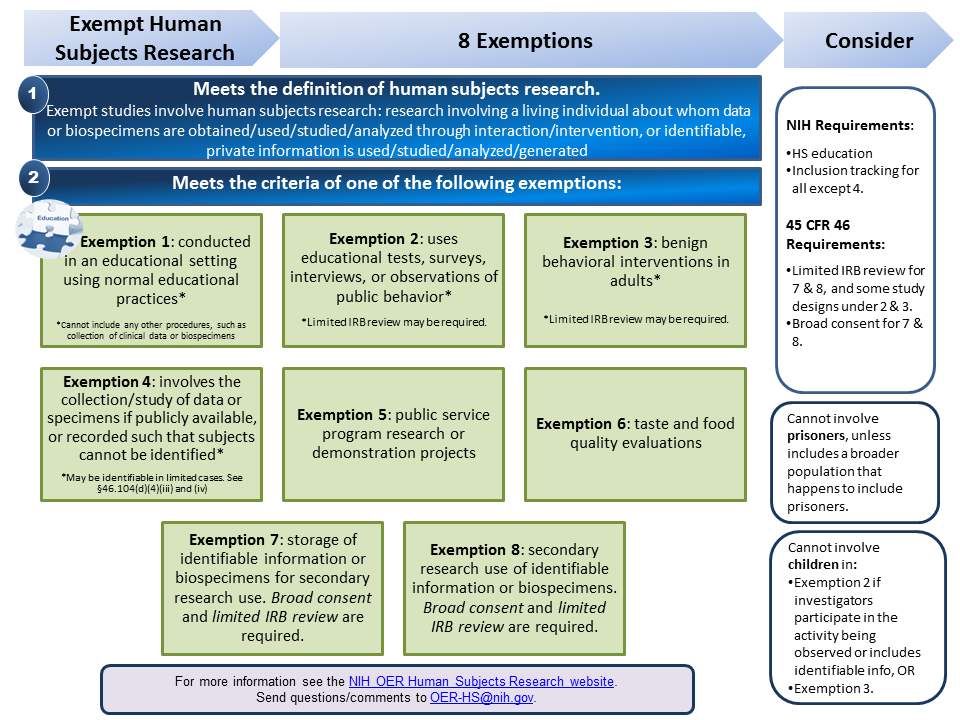
grants.nih.gov › grants › how-to-apply-applicationG. 500 - PHS Human Subjects and Clinical Trials Information Note for studies involving only the secondary use of identifiable biospecimens or data: For studies where the only involvement of human subjects is the use of identifiable biospecimens or data originally collected for another purpose, complete the PHS Human Subjects and Clinical Trials Information form with information specific to the current study and not the original collection unless the ... › Standards-Ethics › InstitutionalIRB FAQs for Survey Researchers - AAPOR According to the Federal regulations (45 CFR 46.101(b)), survey research may be exempt from the regulations unless "the information obtained is recorded in such a manner that the human subjects can be identified, directly or through identifiers linked to the subjects" or if "federal statute(s) require(s) without exception that the ...
grants.nih.gov › grants › how-to-apply-applicationG.500 - PHS Human Subjects and Clinical Trials Information Oct 25, 2021 · Note for studies involving only the secondary use of identifiable biospecimens or data: For studies where the only involvement of human subjects is the use of identifiable biospecimens or data originally collected for another purpose, complete the PHS Human Subjects and Clinical Trials Information form with information specific to the current study and not the original collection unless the ...

Examples of exempt human specimens
› ohrp › regulations-and-policy2018 Requirements (2018 Common Rule) | HHS.gov Authority: 5 U.S.C. 301; 42 U.S.C. 289(a). Editorial Note: The Department of Health and Human Services issued a notice of waiver regarding the requirements set forth in part 46, relating to protection of human subjects, as they pertain to demonstration projects, approved under section 1115 of the Social Security Act, which test the use of cost--sharing, such as deductibles, copayment and ...
Examples of exempt human specimens. › ohrp › regulations-and-policy2018 Requirements (2018 Common Rule) | HHS.gov Authority: 5 U.S.C. 301; 42 U.S.C. 289(a). Editorial Note: The Department of Health and Human Services issued a notice of waiver regarding the requirements set forth in part 46, relating to protection of human subjects, as they pertain to demonstration projects, approved under section 1115 of the Social Security Act, which test the use of cost--sharing, such as deductibles, copayment and ...















Post a Comment for "45 examples of exempt human specimens"