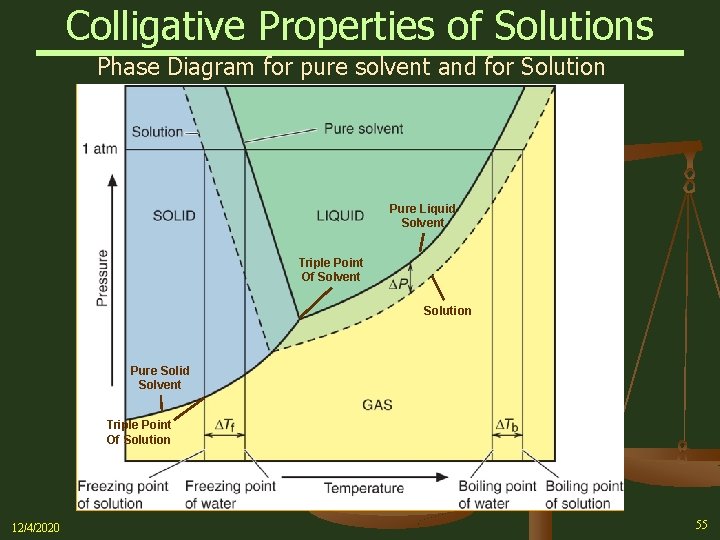
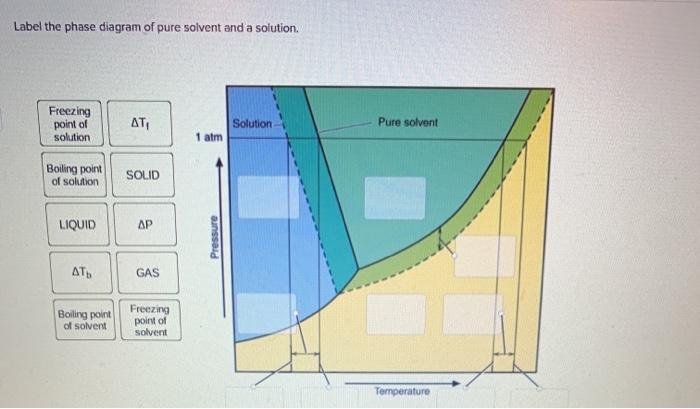
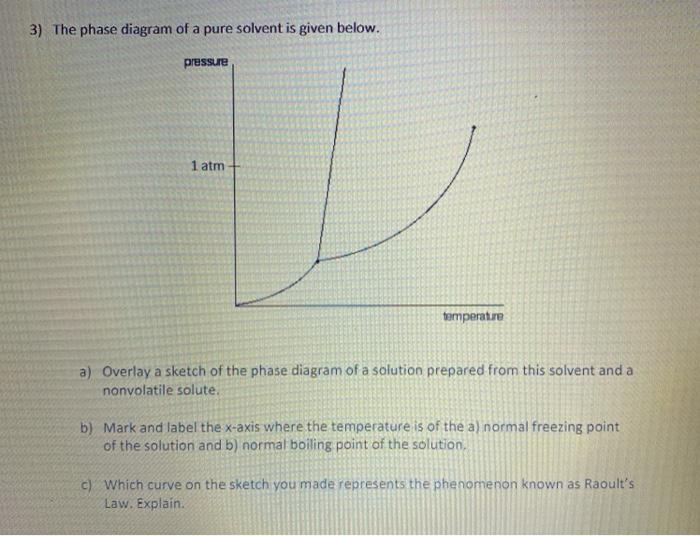
41 label the phase diagram of pure solvent and a solution.
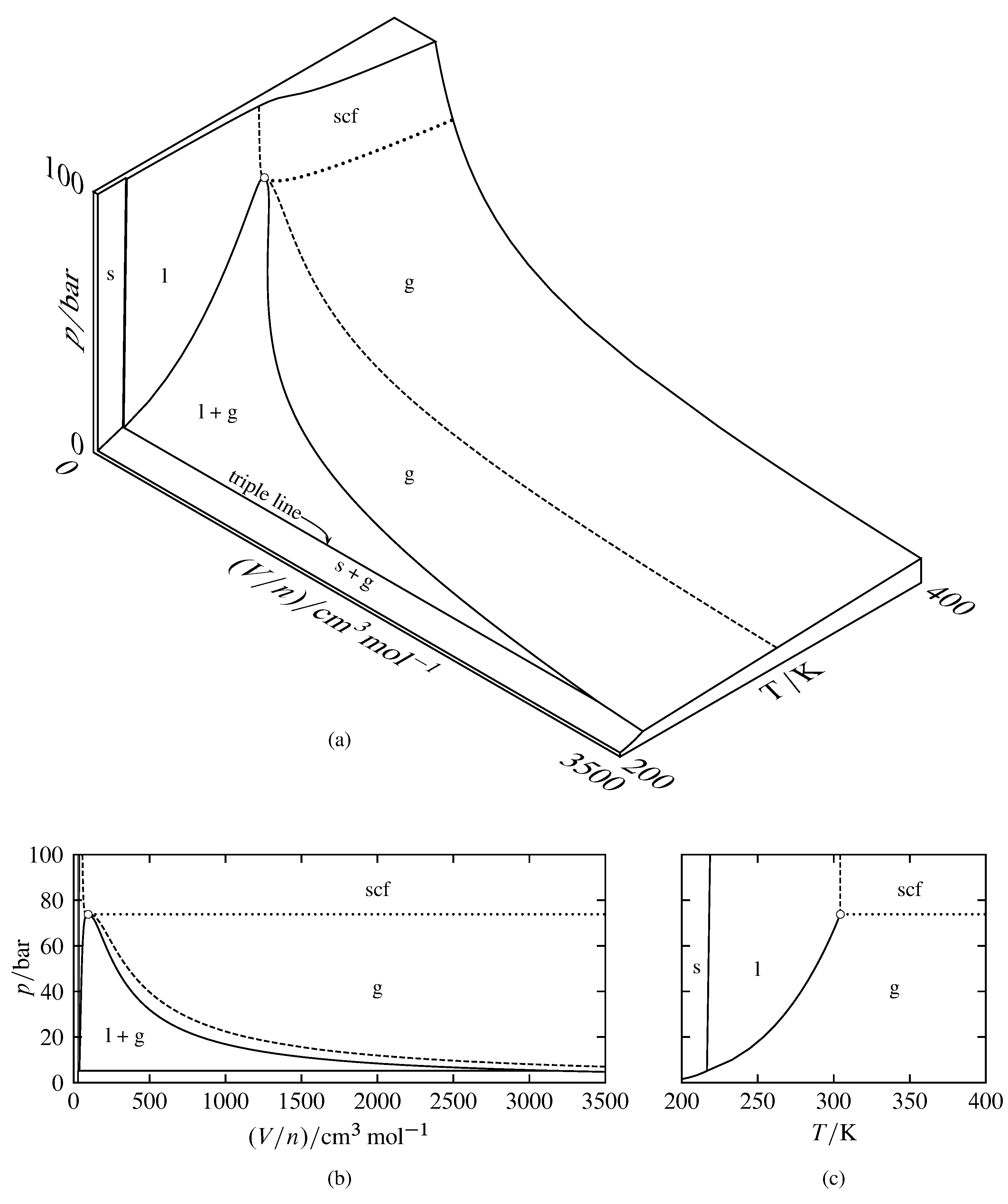
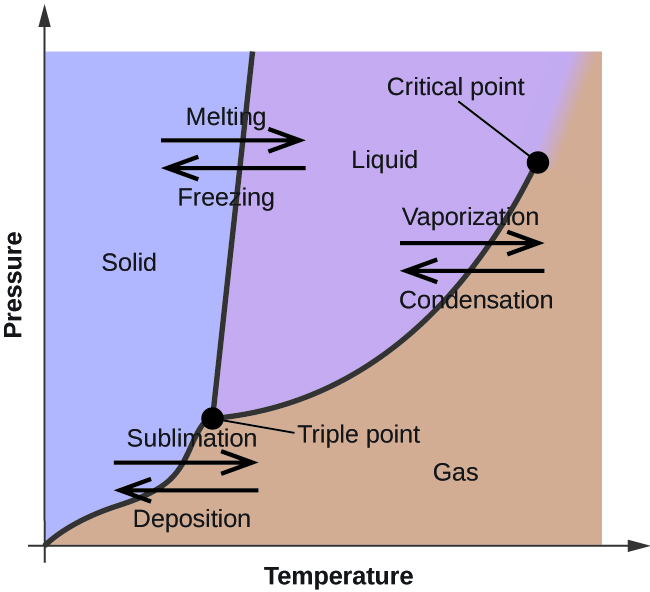
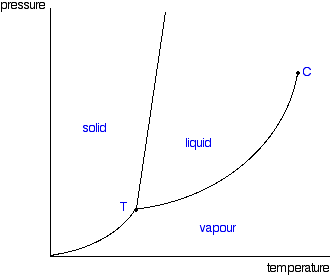
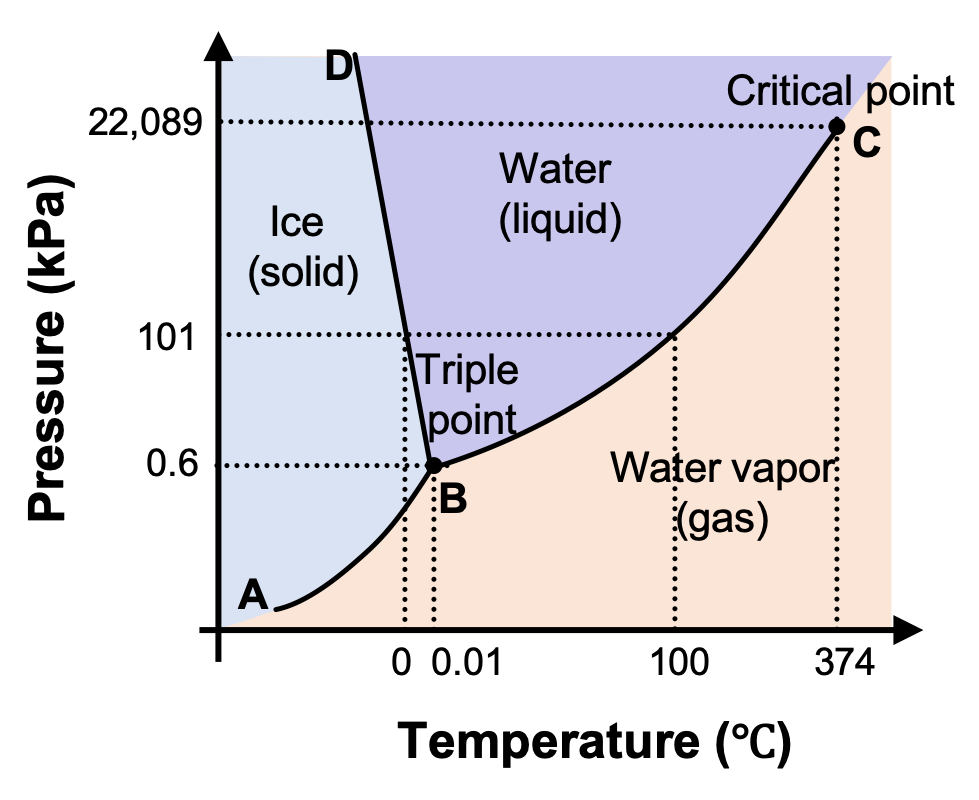
Phase diagram - Wikipedia The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas. The curves on the phase diagram show the points where the free energy (and other derived properties) becomes non-analytic: their derivatives with respect to the coordinates (temperature and pressure in this example) change discontinuously (abruptly). Phase Diagrams | Chemistry for Majors - Lumen Learning Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation ...
Question : Label the phase diagram of pure solvent and a solution. - Chegg Expert Answer. 100% (137 ratings) This is …. View the full answer. Transcribed image text: Label the phase diagram of pure solvent and a solution.
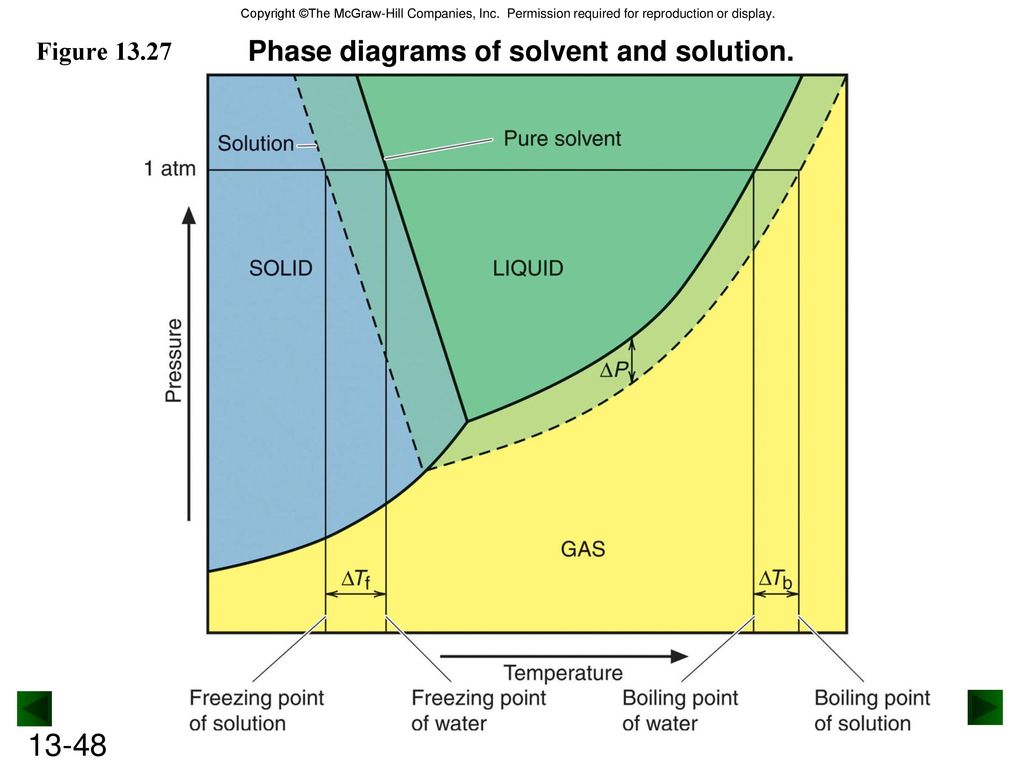
Label the phase diagram of pure solvent and a solution.
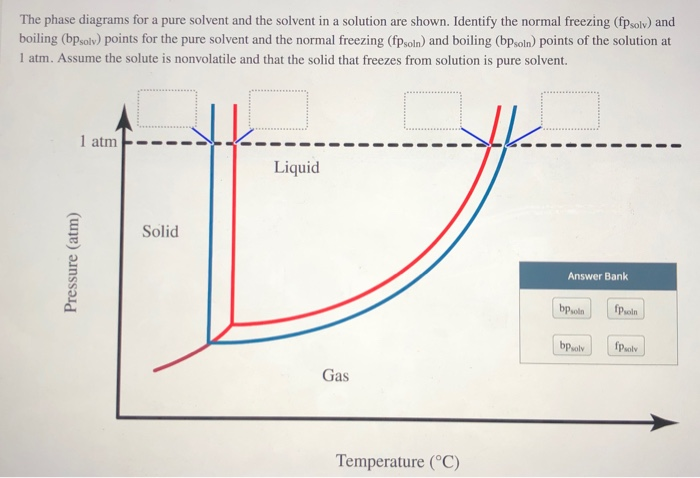
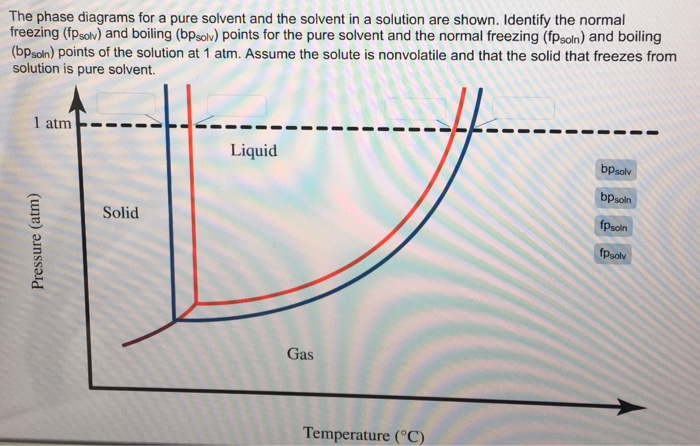
Answered: The phase diagrams for a pure solvent… | bartleby The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm Liquid Solid Answer Bank fpsolv bpsolv fpsoln bpsoln Gas Temperature (°C) Pressure (atm) PDF Phase Diagrams, Solid Solutions, Phase Transformations phase diagram A(1100, 60): 1 phase: B(1250, 35): 2 phases: L + Determination of phase(s) present Melting points: Cu = 1085°C, Ni = 1453 °C Solidus - Temperature where alloy is completely solid. Above this line, liquefaction begins. Liquidus - Temperature where alloy is completely liquid. Below this line, solidification begins. Phase Diagrams – Chemistry - University of Hawaiʻi A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, boiling points). A typical phase diagram for a pure substance is shown in .
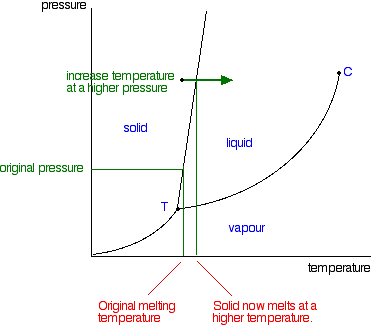
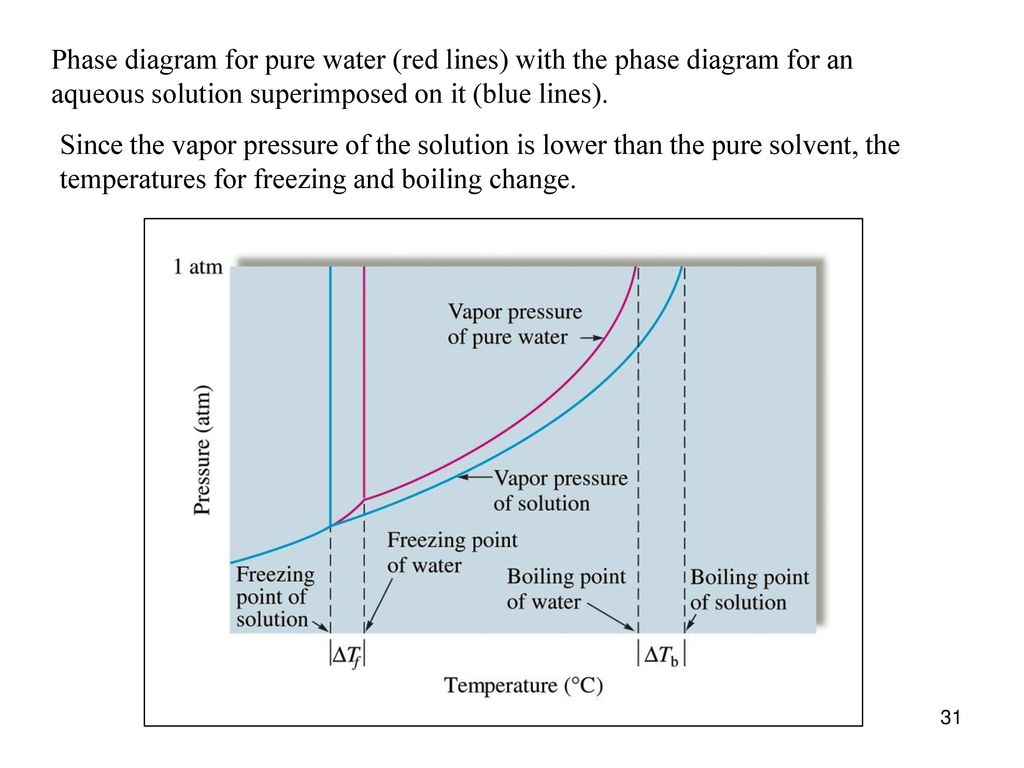
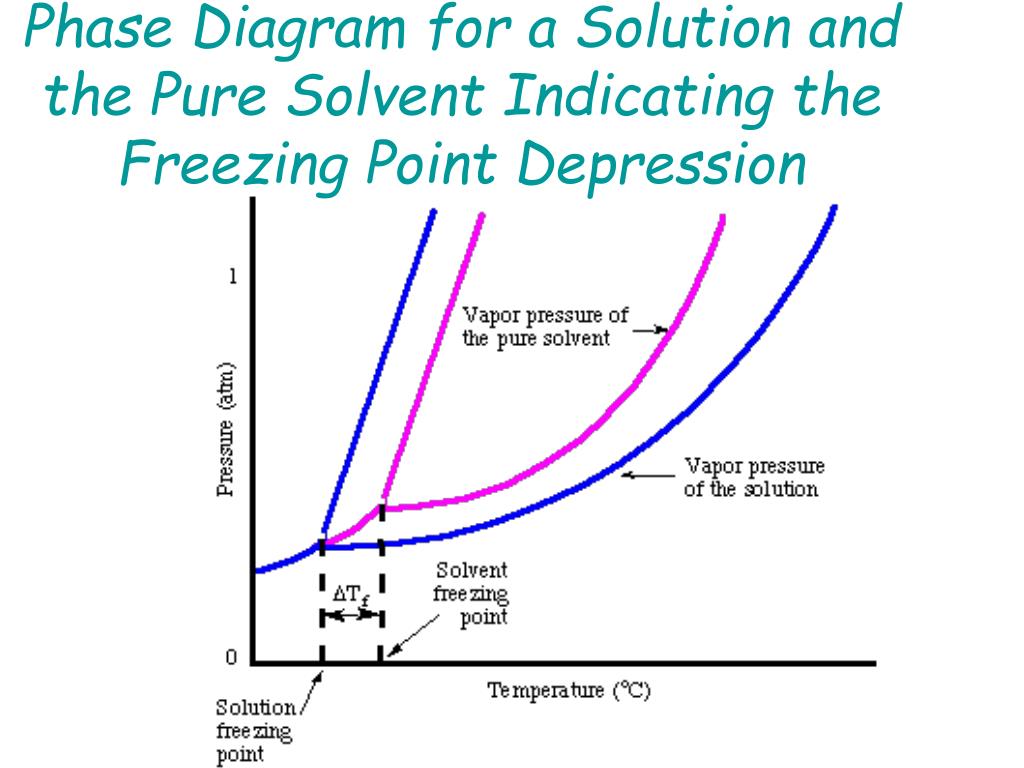
Label the phase diagram of pure solvent and a solution.. An overview on the use of additives and preparation procedure in phase … In the context of long-term energy storage, solid-to-solid PCMs are recently garnering considerable attention owing to the discovery of materials, mainly plastic crystals, with high enthalpy of transition between the solid phases .They avoid the liquid phase and offer several advantages in comparison to the solid-to-liquid PCMs, such as lack of leakages and of … 57 Features of Phase Diagrams (M11Q1) - Unizin A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. 2.2: Molecular Weight Determination - Chemistry LibreTexts Mar 21, 2021 · In Equation \ref{1} the freezing point depression of a non-ionic solution is described. Where ∆T f is the change in the initial and final temperature of the pure solvent, K f is the freezing point depression constant for the pure solvent, and m (moles solute/kg solvent) is the molality of the solution. \[\Delta T _ { f } = K _ { f } m \label{1}\] phase diagrams of pure substances - chemguide Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram. Under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. At 2, it would be a liquid; and at 3, it would be a vapour (a gas).
Phase Diagrams | Chemistry - Lumen Learning A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, boiling points). A typical phase diagram for a pure substance is shown in Figure 1. PDF Phase Diagrams - site.iugaza.edu.ps The completely solidified alloy in the phase diagram shown is a solid solution because: Alloying element (Cu, solute) is completely dissolved in host metal (Ni, solvent) Each grain has same composition Atomic radius of Cu is 0.128nm & that of Ni is 0.125nm, Both elements are FCC; HRRs are obeyed. 12/3/2013 11:12 PM The phase diagram for solvent and solutions is shown in the figure ... The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A. A. B. B. C. C. D. D. Hard. Open in App. Solution. Verified by Toppr. Correct option is D) ... The phase diagram for a pure substance is shown above. Phase Diagrams | Boundless Chemistry | | Course Hero A phase diagram is a graph which shows under what conditions of temperature and pressure distinct phases of matter occur. The simplest phase diagrams are of pure substances. These diagrams plot pressure on the y-axis and temperature on the x-axis. Although phases are conceptually simple, they are difficult to define precisely.
Solved Label the phase diagram of pure solvent and a | Chegg.com Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP ... PDF Phase Diagrams A phase diagram is a graphical representation of the physical states of a substance as they relate to temperature and pressure (Figure 1). A typical phase diagram has pressure on the y-axis and temperature on the x-axis. Figure 1: Example of a general phase diagram. The labels on the graph represent the physical state or phase of the substance at equilibrium Phase Diagram - SlideShare - The phase diagram shows two distinct phases; one is liquid metal solution and the other is solid solution. - Liquidus is that line - Above which the alloy is in liquid state - Where solidification starts - Solidus is that - Below which the alloy is in solid state, and - Where the solidification completes. solid-liquid phase diagrams: salt solution - chemguide The phase diagram for sodium chloride solution. What the phase diagram looks like. ... because adding a non-volatile solute to a solvent increases its boiling point. ... you could read the page about the phase diagrams of pure substances. Again, it isn't essential to understanding the rest of this page. You just need to be aware that the line ...
Answered: 1) Label the LLE diagram provided… | bartleby 1) Label the LLE diagram provided indicating which component is the solvent and which is cariet, and identify the solvent rich (extract) phase boundary and the raffinate phase boundary. 2) Identify Each of the (mark as 'a', 'b', etc) following points and indicate types and number of phases present in each composition.
Patricia LOSADA-PÉREZ | Professor | Université Libre de Bruxelles ... The critical region in the phase diagram of condensed matter systems such as fluids or fluid mixtures is characterized by the anomalous behaviour of specific thermodynamic properties.
Phase Diagram | Explanation, Definition, Summary & Facts The phase diagram of a substance can be used to identify the physical and chemical properties of that substance. Here, we will study a general phase diagram by considering different values of one variable while keeping the other variable value constant. In a phase diagram temperature values are drawn on x-axis, whereas pressure values on y-axis.
10.4 Phase Diagrams - Chemistry Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation ...
1.2 3 Separation Techniques - Save My Exams The solution is heated, and pure water evaporates producing a vapour which rises through the neck of the round bottomed flask; The vapour passes through the condenser, where it cools and condenses, turning into the pure liquid that is collected in a beaker ; After all the water is evaporated from the solution, only the solid solute will be left behind; Diagram showing the …
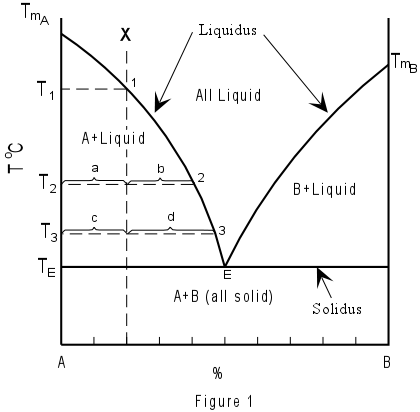
Binary Solid-Liquid Phase Diagram | Chem Lab For more information on binary solid-liquid phase diagrams and the derivation of these equations, please see the references. 1-4 . From your data, generate the solid-liquid phase diagram for your system and determine Δ fus H and the melting point for the two pure substances at 95% confidence. Determine the eutectic temperature and composition ...
10.4 Phase Diagrams - General Chemistry 1 & 2 Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation ...
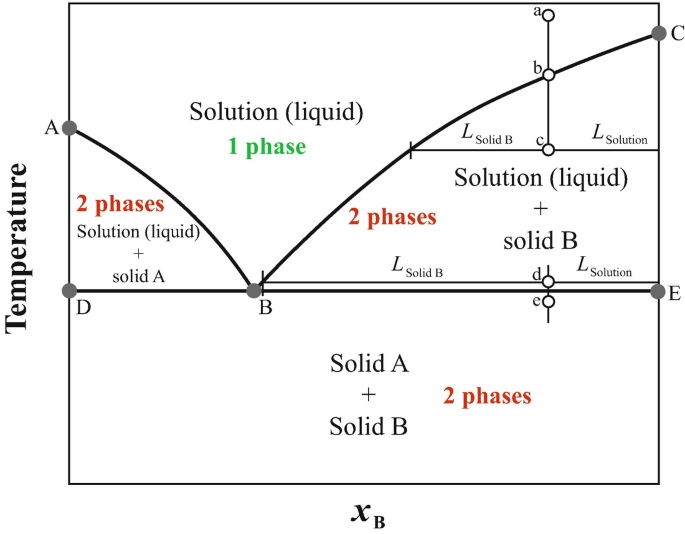
Chapter 8 Phase Diagrams - Central Michigan University Example 8.2 Interpreting a liquid-liquid phase diagram . A mixture of 50 g of hexane (0.59 mol) and 50 g nitobenzene (0.41 mol) was prepared at 290 K. ... Pure solid B begins to come out of solution and the remaining liquid becomes richer in A. (2) a 2 ® a 3. More of the solid forms, and the relative amounts of the solid and liquid (which are ...
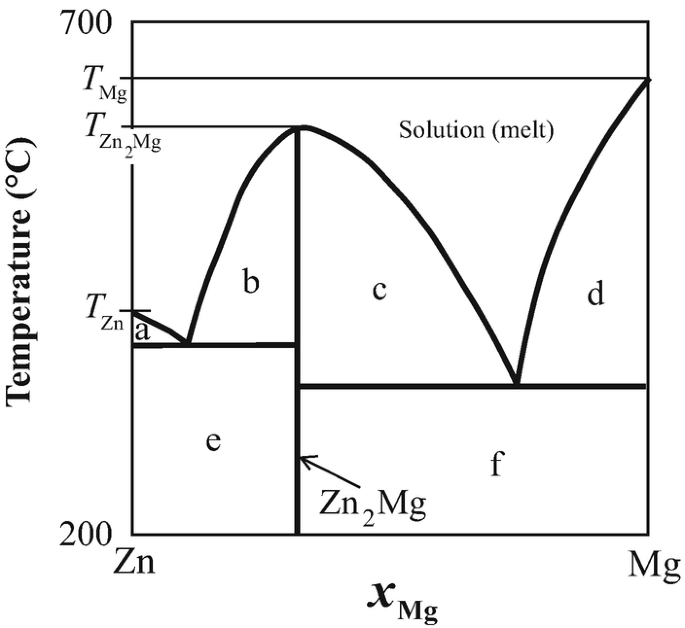
PDF Phase Diagrams - site.iugaza.edu.ps The completely solidified alloy in the phase diagram shown is a solid solution because: Alloying element (Cu, solute) is completely dissolved in host metal (Ni, solvent) Each grain has same composition Atomic radius of Cu is 0.128nm & that of Ni is 0.125nm, Both elements are FCC; HRRs are obeyed. 1/2/2015 1:48 PM
Ternary Phase Diagram - an overview | ScienceDirect Topics Ternary phase diagrams are used to represent all possible mixtures of three solvents [1]; they are described in Chapter 3.Here, we shall indicate how they should be used to minimize the solvent consumption. Figure 2.1 (top) shows the methanol–chloroform–water ternary phase diagram with the tie-lines in the biphasic domain. Five particular compositions are shown in the …
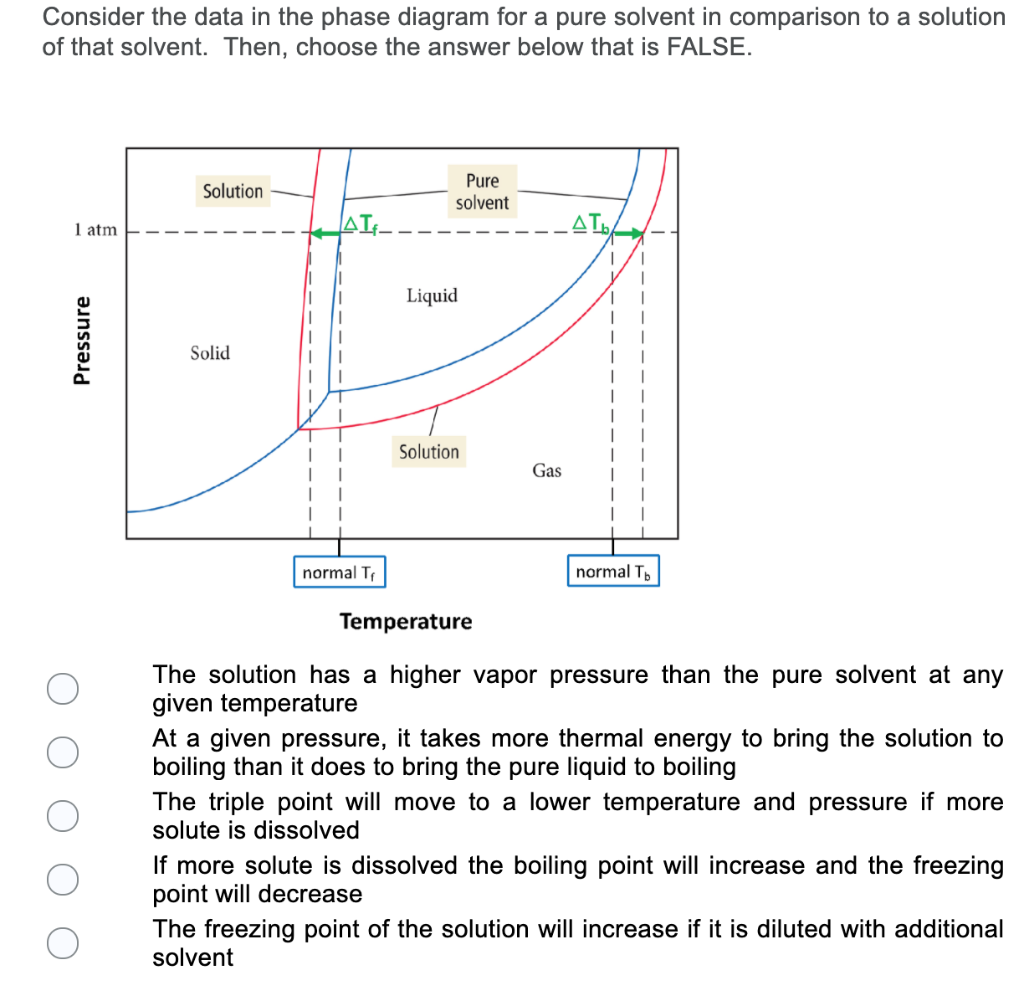
The figure shows two phase diagrams, one for a pure liquid (black line ... Now consider the solution made using the pure liquid substance as the solvent, with the phase diagram indicated by the red curves. Similarly, the normal boiling point of the pure substance at constant pressure is indicated by C, and the raised normal boiling point of the solution with the pure liquid as the solvent (again, due to addition of nonvolatile solute to the pure liquid substance) is indicated by D, since T ↑ rightwards.
CH103 – Chapter 8: Homeostasis and Cellular Function – Chemistry 8.1 The Concept of Homeostasis. Homeostasis refers to the body’s ability to physiologically regulate its inner environment to ensure its stability in response to fluctuations in external or internal conditions.The liver, the pancreas, the kidneys, and the brain (hypothalamus, the autonomic nervous system and the endocrine system) help maintain homeostasis.
CHAPTER 12, 13, & 14 study guide. Flashcards | Quizlet A solution containing 0.102 g of an unknown compound dissolved in 100. mL of water has an osmotic pressure of 28.1 mmHg at 20.°C. What is the molar mass of the compound? (R = 0.08206 L • atm/K • mol, 1 atm = 760 mmHg) ... Label the phase diagram of pure solvent and a solution....
Phase Diagrams - Purdue University Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid ...
CH150: Chapter 7 – Solutions – Chemistry - Western Oregon … 7.1 Introduction: Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. Solutions are all around us. Air, for example, is a solution. If you live near a lake, a river, or an ocean, that body of water is not pure H 2 O but most probably a solution.
Avery 2x2 Label Template - hopper.threestrikesandout.com Label The Phase Diagram Of Pure Solvent And A Solution Label The Phase Diagram Of Pure Solvent And A Solution; Magazine Article Ks2 Jungle Rousseau Henri Ks2 Rainforest Kunstunterricht Matisse Lesson Mural Fore Describe Madagascar Dschungel Grundschule Klimt; Vegetable Egg Foo Young Foo Egg Young Recipe Homemade Takeout Better Than
Liquid/Solid Phase Diagram - an overview | ScienceDirect Topics Construct the binary (solid + liquid) phase diagram and label all the regions. (b) Use the (T, x 2) data near x 2 = 0 and x 2 = 1 to calculate Δ fus H m for both components. Assume this Δ fus H m is constant with temperature and calculate the ideal freezing point at every 0.1 mole fraction unit. Plot these results on the diagram constructed in part (a) to compare the actual freezing points with the ideal solution prediction.
Phase Diagrams - Lardbucket.org Remember that a phase diagram, such as the one in Figure 11.22 "A Typical Phase Diagram for a Substance That Exhibits Three Phases—Solid, Liquid, and Gas—and a Supercritical Region", is for a single pure substance in a closed system, not for a liquid in an open beaker in contact with air at 1 atm pressure. In practice, however, the conclusions reached about the behavior of a substance in a closed system can usually be extrapolated to an open system without a great deal of error.
Rearrangement - Michigan State University This ion is commonly seen in the mass spectrum of methane (gas phase), but decomposes in solution as a consequence of its extreme acidity. To its right are two larger non-classical ions, 2-norbornyl and 7-norbornenyl. A pentacoordinate carbon atom is identified in each case. Resonance contributors to these ions are shown to the right of the dashed bond …
Ethanol - Wikipedia Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic chemical compound.It is a simple alcohol with the chemical formula C 2 H 6 O. Its formula can be also written as CH 3 − CH 2 − OH or C 2 H 5 OH (an ethyl group linked to a hydroxyl group), and is often abbreviated as EtOH.Ethanol is a volatile, flammable, colorless liquid with a ...
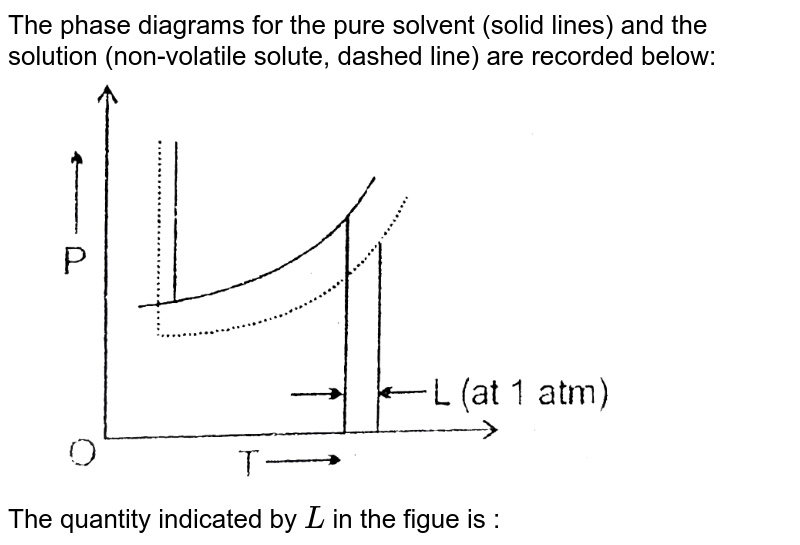
The phase diagrams for the pure solvent (solid lines) and the solution ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by `L` i...
Phase Diagrams – Chemistry - University of Hawaiʻi A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, boiling points). A typical phase diagram for a pure substance is shown in .
PDF Phase Diagrams, Solid Solutions, Phase Transformations phase diagram A(1100, 60): 1 phase: B(1250, 35): 2 phases: L + Determination of phase(s) present Melting points: Cu = 1085°C, Ni = 1453 °C Solidus - Temperature where alloy is completely solid. Above this line, liquefaction begins. Liquidus - Temperature where alloy is completely liquid. Below this line, solidification begins.
Answered: The phase diagrams for a pure solvent… | bartleby The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm Liquid Solid Answer Bank fpsolv bpsolv fpsoln bpsoln Gas Temperature (°C) Pressure (atm)















Post a Comment for "41 label the phase diagram of pure solvent and a solution."